Psychedelic drug
| Part of a series on |
| Psychedelia |
|---|
 |

Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary mental states (known as psychedelic experiences or "trips") and a perceived "expansion of consciousness".[2][3] Also referred to as classic hallucinogens or serotonergic hallucinogens, the term psychedelic is sometimes used more broadly to include various types of hallucinogens, such as those which are atypical or adjacent to psychedelia like salvia and MDMA, respectively.[4]
Classic psychedelics generally cause specific psychological, visual, and auditory changes, and oftentimes a substantially altered state of consciousness.[5][6] They have had the largest influence on science and culture, and include mescaline, LSD, psilocybin, and DMT.[7][8]
Most psychedelic drugs fall into one of the three families of chemical compounds: tryptamines, phenethylamines, or lysergamides (LSD is considered both a tryptamine and lysergamide). They act via serotonin 2A receptor agonism.[2][9][10][11][4] When compounds bind to serotonin 5-HT2A receptors,[12] they modulate the activity of key circuits in the brain involved with sensory perception and cognition. However, the exact nature of how psychedelics induce changes in perception and cognition via the 5-HT2A receptor is still unknown.[13] The psychedelic experience is often compared to non-ordinary forms of consciousness such as those experienced in meditation,[14][3] mystical experiences,[6][5] and near-death experiences,[5] which also appear to be partially underpinned by altered default mode network activity.[15] The phenomenon of ego death is often described as a key feature of the psychedelic experience.[14][3][5]
Many psychedelic drugs are illegal to possess without lawful authorisation, exemption or license worldwide under the UN conventions, with occasional exceptions for religious use or research contexts. Despite these controls, recreational use of psychedelics is common.[16][17] Legal barriers have made the scientific study of psychedelics more difficult. Research has been conducted, however, and studies show that psychedelics are physiologically safe and rarely lead to addiction.[18][19] Studies conducted using psilocybin in a psychotherapeutic setting reveal that psychedelic drugs may assist with treating depression, alcohol addiction, and nicotine addiction.[11][20] Although further research is needed, existing results suggest that psychedelics could be effective treatments for certain forms of psychopathology.[21][22][23][17] A 2022 survey found that 28% of Americans had used a psychedelic at some point in their life.[24]
Etymology and nomenclature
[edit]
The term psychedelic was coined by the psychiatrist Humphrey Osmond during written correspondence with author Aldous Huxley (written in a rhyme: “To fathom Hell or soar angelic/Just take a pinch of psychedelic.”[25]) and presented to the New York Academy of Sciences by Osmond in 1957.[26] It is irregularly[27] derived from the Greek words ψυχή (psychḗ, meaning 'mind, soul') and δηλείν (dēleín, meaning 'to manifest'), with the intended meaning "mind manifesting" or alternatively "soul manifesting", and the implication that psychedelics can reveal unused potentials of the human mind.[28] The term was loathed by American ethnobotanist Richard Schultes but championed by American psychologist Timothy Leary.[29]
Aldous Huxley had suggested his own coinage phanerothyme (Greek phaneroein- "to make manifest or visible" and Greek thymos "soul", thus "to reveal the soul") to Osmond in 1956.[30] Recently, the term entheogen (meaning "that which produces the divine within") has come into use to denote the use of psychedelic drugs, as well as various other types of psychoactive substances, in a religious, spiritual, and mystical context.[31]
In 2004, David E. Nichols wrote the following about the nomenclature used for psychedelic drugs:[31]
Many different names have been proposed over the years for this drug class. The famous German toxicologist Louis Lewin used the name phantastica earlier in this century, and as we shall see later, such a descriptor is not so farfetched. The most popular names—hallucinogen, psychotomimetic, and psychedelic ("mind manifesting")—have often been used interchangeably. Hallucinogen is now, however, the most common designation in the scientific literature, although it is an inaccurate descriptor of the actual effects of these drugs. In the lay press, the term psychedelic is still the most popular and has held sway for nearly four decades. Most recently, there has been a movement in nonscientific circles to recognize the ability of these substances to provoke mystical experiences and evoke feelings of spiritual significance. Thus, the term entheogen, derived from the Greek word entheos, which means "god within", was introduced by Ruck et al. and has seen increasing use. This term suggests that these substances reveal or allow a connection to the "divine within". Although it seems unlikely that this name will ever be accepted in formal scientific circles, its use has dramatically increased in the popular media and on internet sites. Indeed, in much of the counterculture that uses these substances, entheogen has replaced psychedelic as the name of choice and we may expect to see this trend continue.
Robin Carhart-Harris and Guy Goodwin write that the term psychedelic is preferable to hallucinogen for describing classical psychedelics because of the term hallucinogen's "arguably misleading emphasis on these compounds' hallucinogenic properties."[32]
While the term psychedelic is most commonly used to refer only to serotonergic hallucinogens,[11][10][33][34] it is sometimes used for a much broader range of drugs, including empathogen–entactogens, dissociatives, and atypical hallucinogens/psychoactives such as Amanita muscaria, Cannabis sativa, Nymphaea nouchali and Salvia divinorum.[22][35] Thus, the term serotonergic psychedelic is sometimes used for the narrower class.[36][37] It is important to check the definition of a given source.[31] This article uses the more common, narrower definition of psychedelic.
Examples
[edit]

- 2C-B (2,5-dimethoxy-4-bromophenethylamine) is a substituted phenethylamine first synthesised in 1974 by Alexander Shulgin.[38][page needed] 2C-B is both a psychedelic and a mild entactogen, with its psychedelic effects increasing and its entactogenic effects decreasing with dosage. 2C-B is the most well known compound in the 2C family, their general structure being discovered as a result of modifying the structure of mescaline.[38][page needed]
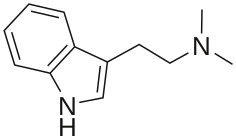
- DMT (N,N-dimethyltryptamine) is an indole alkaloid found in various species of plants. Traditionally it is consumed by tribes in South America in the form of ayahuasca. A brew is used that consists of DMT-containing plants as well as plants containing MAOIs, specifically harmaline, which allows DMT to be consumed orally without being rendered inactive by monoamine oxidase enzymes in the digestive system.[39] In the Western world DMT is more commonly consumed via the vaporisation of freebase DMT. Whereas Ayahuasca typically lasts for several hours, inhalation has an onset measured in seconds and has effects measured in minutes, being significantly more intense.[40] Particularly in vaporised form, DMT has the ability to cause users to enter a hallucinatory realm fully detached from reality, being typically characterised by hyperbolic geometry, and described as defying visual or verbal description.[41] Users have also reported encountering and communicating with entitites within this hallucinatory state.[42] DMT is the archetypal substituted tryptamine, being the structural scaffold of psilocybin and – to a lesser extent – the lysergamides.
- LSD (Lysergic acid diethylamide) is a derivative of lysergic acid, which is obtained from the hydrolysis of ergotamine. Ergotamine is an alkaloid found in the fungus Claviceps purpurea, which primarily infects rye. LSD is both the prototypical psychedelic and the prototypical lysergamide. As a lysergamide, LSD contains both a tryptamine and phenethylamine group within its structure. As a result of containing a phenethylamine group LSD agonises dopamine receptors as well as serotonin receptors,[43] making it more energetic in effect in contrast to the more sedating effects of psilocin, which is not a dopamine agonist.[44]
- Mescaline (3,4,5-trimethoxyphenethylamine) is a phenethylamine alkaloid found in various species of cacti, the best-known of these being peyote (Lophophora williamsii) and the San Pedro cactus (Trichocereus macrogonus var. pachanoi, syn. Echinopsis pachanoi). Mescaline has effects comparable to those of LSD and psilocybin, albeit with a greater emphasis on colors and patterns.[45][page needed] Ceremonial San Pedro use seems to be characterized by relatively strong spiritual experiences, and low incidence of challenging experiences.[46]
- Psilocin (4-HO-DMT) is the dephosphorylated active metabolite of the indole alkaloid psilocybin and a substituted tryptamine, which is produced in over 200 species of fungi. Of the Classical psychedelics psilocybin has attracted the greatest academic interest regarding its ability to manifest mystical experiences,[47] although all psychedelics are capable of doing so to variable degrees. O-Acetylpsilocin (4-AcO-DMT) is an acetylated analog of psilocin. Additionally, replacement of a methyl group at the dimethylated nitrogen with an isopropyl or ethyl group yields 4-HO-MIPT and 4-HO-MET, respectively.[48]
Uses
[edit]Traditional
[edit]


A number of frequently mentioned or traditional psychedelics such as Ayahuasca (which contains DMT), San Pedro, Peyote, and Peruvian torch (which all contain mescaline), Psilocybe mushrooms (which contain psilocin/psilocybin) and Tabernanthe iboga (which contains the unique psychedelic ibogaine) all have a long and extensive history of spiritual, shamanic and traditional usage by indigenous peoples in various world regions, particularly in Latin America, but also Gabon, Africa in the case of iboga.[49] Different countries and/or regions have come to be associated with traditional or spiritual use of particular psychedelics, such as the ancient and entheogenic use of psilocybe mushrooms by the native Mazatec people of Oaxaca, Mexico[50] or the use of the ayahuasca brew in the Amazon basin, particularly in Peru for spiritual and physical healing as well as for religious festivals.[51] Peyote has also been used for several thousand years in the Rio Grande Valley in North America by native tribes as an entheogen.[52] In the Andean region of South America, the San Pedro cactus (Trichocereus macrogonus var. pachanoi, syn. Echinopsis pachanoi) has a long history of use, possibly as a traditional medicine. Archaeological studies have found evidence of use going back two thousand years, to Moche culture,[53] Nazca culture,[54] and Chavín culture. Although authorities of the Roman Catholic church attempted to suppress its use after the Spanish conquest,[55] this failed, as shown by the Christian element in the common name "San Pedro cactus" – Saint Peter cactus. The name has its origin in the belief that just as St Peter holds the keys to heaven, the effects of the cactus allow users "to reach heaven while still on earth."[56] In 2022, the Peruvian Ministry of Culture declared the traditional use of San Pedro cactus in northern Peru as cultural heritage.[57]
Although people of Western culture have tended to use psychedelics for either psychotherapeutic or recreational reasons, most indigenous cultures, particularly in South America, have seemingly tended to use psychedelics for more supernatural reasons such as divination. This can often be related to "healing" or health as well but typically in the context of finding out what is wrong with the individual, such as using psychedelic states to "identify" a disease and/or its cause, locate lost objects, and identify a victim or even perpetrator of sorcery.[58] In some cultures and regions, even psychedelics themselves, such as ayahuasca and the psychedelic lichen of eastern Ecuador (Dictyonema huaorani) that supposedly contains both 5-MeO-DMT and psilocybin, have also been used by witches and sorcerers to conduct their malicious magic, similarly to nightshade deliriants like brugmansia and latua.[58][citation needed]
Psychedelic therapy
[edit]Psychedelic therapy (or psychedelic-assisted therapy) is the proposed use of psychedelic drugs to treat mental disorders.[59] As of 2021, psychedelic drugs are controlled substances in most countries and psychedelic therapy is not legally available outside clinical trials, with some exceptions.[34][60]
The procedure for psychedelic therapy differs from that of therapies using conventional psychiatric medications. While conventional medications are usually taken without supervision at least once daily, in contemporary psychedelic therapy the drug is administered in a single session (or sometimes up to three sessions) in a therapeutic context.[61] The therapeutic team prepares the patient for the experience beforehand and helps them integrate insights from the drug experience afterwards.[62][63] After ingesting the drug, the patient normally wears eyeshades and listens to music to facilitate focus on the psychedelic experience, with the therapeutic team interrupting only to provide reassurance if adverse effects such as anxiety or disorientation arise.[62][63]
As of 2022, the body of high-quality evidence on psychedelic therapy remains relatively small and more, larger studies are needed to reliably show the effectiveness and safety of psychedelic therapy's various forms and applications.[21][22] On the basis of favorable early results, ongoing research is examining proposed psychedelic therapies for conditions including major depressive disorder,[21][64] and anxiety and depression linked to terminal illness.[21][65] The United States Food and Drug Administration has granted "breakthrough therapy" status, which expedites the assessment of promising drug therapies for potential approval,[note 1] to psilocybin therapy for treatment-resistant depression and major depressive disorder.[34]
Recreational
[edit]Recreational use of psychedelics has been common since the psychedelic era of the mid-1960s and continues to play a role in various festivals and events, including Burning Man.[16][17] A survey published in 2013 found that 13.4% of American adults had used a psychedelic.[67]
A June 2024 report by the RAND Corporation suggests psilocybin mushrooms may be the most prevalent psychedelic drug among adults in the United States. The RAND national survey indicated that 3.1% of U.S. adults reported using psilocybin in the past year. Roughly 12% of respondents acknowledged lifetime use of psilocybin, while a similar percentage reported having used LSD at some point in their lives. MDMA, also known as ecstasy, showed a lower prevalence of use at 7.6%. Notably, less than 1% of U.S. adults reported using any psychedelic drugs within the past month.[68]
Microdosing
[edit]Psychedelic microdosing is the practice of using sub-threshold doses (microdoses) of psychedelics in an attempt to improve creativity, boost physical energy level, emotional balance, increase performance on problems-solving tasks and to treat anxiety, depression and addiction.[69][70] The practice of microdosing has become more widespread in the 21st century with more people claiming long-term benefits from the practice.[71][72]
A 2022 study recognized signatures of psilocybin microdosing in natural language and concluded that low amount of psychedelics have potential for application, and ecological observation of microdosing schedules.[73][74]
Pharmacology
[edit]Mechanism of action
[edit]While the method of action of psychedelics is not fully understood, they are known to show affinities for various 5-HT (serotonin) receptors in different ways and levels, and may be classified by their activity at different 5-HT sub-types, particularly 5-HT1A, 5-HT2A, and 5-HT2C.[31] It is almost unanimously agreed that psychedelics produce their effect by acting as strong partial agonists at the 5-HT2A receptors.[2][9][10][11] How this produces the psychedelic experience is unclear, but it is likely that it acts by increasing excitation in the cortex, possibly by specifically facilitating input from the thalamus, the major relay for sensory information input to the cortex.[31][75] Additionally, researchers discovered that many psychedelics are potent psychoplastogens, compounds capable of promoting rapid and sustained neural plasticity.[76][77]
Psychedelics are thought to mediate their hallucinogenic effects by activation of serotonin 5-HT2A receptors.[78][79] Serotonergic psychedelics, such as the tryptamines psilocin, DMT, and 5-MeO-DMT, the phenethylamines mescaline, DOM, and 2C-B, and ergolines and lysergamides like LSD, all act as agonists of the serotonin 5-HT2A receptors.[80][78][81] In addition, some psychedelics, such as phenethylamines like DOM and 2C-B, show high selectivity for the serotonin 5-HT2 receptors over other serotonin receptors.[78][81] There is a very strong correlation between 5-HT2A receptor affinity and human hallucinogenic potency.[78] In addition, the intensity of hallucinogenic effects in humans is directly correlated with the level of serotonin 5-HT2A receptor occupancy as measured with positron emission tomography (PET) imaging.[78][79] Serotonin 5-HT2A receptor blockade with drugs like the semi-selective ketanserin and the non-selective risperidone can abolish the hallucinogenic effects of psychedelics in humans.[78][79] However, studies with more selective serotonin 5-HT2A receptor antagonists, like pimavanserin, are still needed.[82]
In animals, potency for stimulus generalization to the psychedelic DOM in drug discrimination tests is strongly correlated with serotonin 5-HT2A receptor affinity.[78][79] Non-selective serotonin 5-HT2A receptor antagonists, like ketanserin and pirenperone, and selective serotonin 5-HT2A receptor antagonists, like volinanserin (MDL-100907), abolish the stimulus generalization of psychedelics in drug discrimination tests.[78] Conversely, serotonin 5-HT2B and 5-HT2C receptor antagonists are ineffective.[78] The potencies of serotonin 5-HT2 receptor antagonists in blocking psychedelic substitution are strongly correlated with their serotonin 5-HT2A receptor affinities.[78] Highly selective serotonin 5-HT2A receptor agonists have recently been developed and show stimulus generalization to psychedelics, whereas selective serotonin 5-HT2C receptor agonists do not do so.[78] The head-twitch response (HTR) is induced by serotonergic psychedelics and is a behavioral proxy of psychedelic-like effects in animals.[78][83] The HTR is invariably induced by serotonergic psychedelics, is blocked by selective serotonin 5-HT2A receptor antagonists, and is abolished in serotonin 5-HT2A receptor knockout mice.[78][79] In addition, there is a strong correlation between hallucinogenic potency in humans and potency in the HTR assay.[79] Moreover, the HTR paradigm is one of the only animal tests that can distinguish between hallucinogenic serotonin 5-HT2A receptor agonists and non-hallucinogenic serotonin 5-HT2A receptor agonists, such as lisuride.[78] In accordance with the preceding animal and human findings, it has been said that the evidence that the serotonin 5-HT2A receptor mediates the hallucinogenic effects of serotonergic psychedelics is overwhelming.[79]
The serotonin 5-HT2A receptor activates several downstream signaling pathways.[79][84][85] These include the Gq, β-arrestin2, and other pathways.[79][85] Activation of both the Gq and β-arrestin2 pathways have been implicated in mediating the hallucinogenic effects of serotonergic psychedelics.[79][84][86] However, subsequently, activation of the Gq pathway and not β-arrestin2 has been implicated.[85][84][86][87][88] Interestingly, Gq signaling appeared to mediate hallucinogenic-like effects, whereas β-arrestin2 mediated receptor downregulation and tachyphylaxis.[85][88] The lack of psychedelic effects with non-hallucinogenic serotonin 5-HT2A receptor agonists may be due to partial agonism of the serotonin 5-HT2A receptor with efficacy insufficient to produce psychedelic effects or may be due to biased agonism of the serotonin 5-HT2A receptor.[78] There appears to be a threshold level of Gq activation (in terms of intrinsic activity, with Emax >70%) required for production of hallucinogenic effects.[81][86][88] Full agonists and partial agonists above this threshold are psychedelic 5-HT2A receptor agonists, whereas partial agonists below this threshold, such as lisuride, 2-bromo-LSD, 6-fluoro-DET, 6-MeO-DMT, and Ariadne, are non-hallucinogenic 5-HT2A receptor agonists.[81][88][89][90][91] In addition, biased agonists that activate β-arrestin2 signaling but not Gq signaling, such as ITI-1549, IHCH-7086, and 25N-N1-Nap, are non-hallucinogenic serotonin 5-HT2A receptor agonists.[81][88][92]
The hallucinogenic effects of serotonergic psychedelics may be critically mediated by serotonin 5-HT2A receptor activation in the medial prefrontal cortex (mPFC).[78] Activation of serotonin 5-HT2A receptors in this area results in marked excitatory and inhibitory effects as well as increased release of glutamate and GABA.[78] Direct injection of serotonin 5-HT2A receptor agonists into the mPFC produces the HTR.[78] Drugs that suppress glutamatergic activity in the mPFC, including AMPA receptor antagonists, metabotropic glutamate mGlu2/3 receptor agonists, μ-opioid receptor agonists, and adenosine A1 receptor agonists, block or suppress many of the neurochemical and behavioral effects of serotonergic psychedelics, including the HTR.[78] Metabotropic glutamate mGlu2 receptors are primarily expressed as presynaptic autoreceptors and may have inhibitory effects on glutamate release.[78] Serotonergic psychedelics have been found to produce frontal cortex hyperactivity in humans in PET and single-photon emission computed tomography (SPECT) imaging studies.[78] The PFC projects to many other cortical and subcortical brain areas, such as the locus coeruleus, nucleus accumbens, and amygdala, among others, and activation of the PFC by serotonergic psychedelics may thereby indirectly modulate these areas.[78] In addition to the PFC, there is moderate to high expression of serotonin 5-HT2A receptors in the primary visual cortex (V1), as well as expression of the serotonin 5-HT2A receptor in other visual areas, and activation of these receptors may contribute to or mediate the visual effects of serotonergic psychedelics.[78][79][93][94][95] Serotonergic psychedelics also directly or indirectly modulate a variety of other brain areas, and this may be involved in their effects as well.[79]
Although the hallucinogenic effects of serotonergic psychedelics are thought to be mediated by serotonin 5-HT2A receptor activation, interactions with other receptors, such as the serotonin 5-HT1A and 5-HT2C receptors, may modulate and additionally contribute to their effects.[78][96]
Tryptamines
[edit]
Tryptamine, along with other trace amines, is found in the central nervous system of mammals. It is hypothesized to play a role as a neuromodulator on classical monoamine neurotransmitters, such as dopamine, serotonin, norepinephrine and epinephrine. Tryptamine acts as a non-selective serotonin receptor agonist to activate serotonin receptors, and a serotonin–norepinephrine–dopamine releasing agent (SNDRA) to release more monoamine neurotransmitter, with a preference for evoking serotonin and dopamine release over norepinephrine (epinephrine) release.[97][98][99] Psychedelic tryptamines found in nature include psilocin, DMT, 5-MeO-DMT, and tryptamines that have been synthesized in the laboratory include 4-HO-MET,[100] 4-HO-MiPT,[48] and 5-MeO-DALT.[101]

Phenethylamines
[edit]Phenethylamine is also a trace amine but to a lesser extent acts as a neurotransmitter in the human central nervous system (CNS). Phenethylamine instead regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1), which plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons.[102][103] When VMAT2 is inhibited monoamine neurotransmitters such as dopamine cannot be released into the synapse via typical release mechanisms.[104] Mescaline is a naturally occurring psychedelic protoalkaloid of the substituted phenethylamine class.
Lysergamides
[edit]
Amides of lysergic acid are collectively known as lysergamides, and include a number of compounds with potent agonist and/or antagonist activity at various serotonin and dopamine receptors. The structure of lysergamides contains the structure of both tryptamines and phenethylamines. LSD (Lysergic Acid Diethylamide) is one of many lysergamides. A wide range of lysergamides have emerged in recent years, inspired by existing scientific literature. Others, have appeared from chemical research.[105] 1P-LSD is a derivative and functional analogue of LSD and a homologue of ALD-52. It modifies the LSD molecule by adding a propionyl group to the nitrogen atom of LSD's indole.[106]
Psychedelic experiences
[edit]Although several attempts have been made, starting in the 19th and 20th centuries, to define common phenomenological structures of the effects produced by classic psychedelics, a universally accepted taxonomy does not yet exist.[107][108] At lower doses, features of psychedelic experiences include sensory alterations, such as the warping of surfaces, shape suggestibility, pareidolia and color variations. Users often report intense colors that they have not previously experienced, and repetitive geometric shapes or form constants are common as well. Higher doses often cause intense and fundamental alterations of sensory (notably visual) perception, such as synesthesia or the experience of additional spatial or temporal dimensions.[109] Tryptamines are well documented to cause classic psychedelic states, such as increased empathy, visual distortions (drifting, morphing, breathing, melting of various surfaces and objects), auditory hallucinations, ego dissolution or ego death with high enough dose, mystical, transpersonal and spiritual experiences, autonomous "entity" encounters, time distortion, closed eye hallucinations and complete detachment from reality with a high enough dose.[110] Luis Luna describes psychedelic experiences as having a distinctly gnosis-like quality, and says that they offer "learning experiences that elevate consciousness and can make a profound contribution to personal development."[111] Czech psychiatrist Stanislav Grof studied the effects of psychedelics like LSD early in his career and said of the experience, that it commonly includes "complex revelatory insights into the nature of existence… typically accompanied by a sense of certainty that this knowledge is ultimately more relevant and 'real' than the perceptions and beliefs we share in everyday life."[citation needed] Traditionally, the standard model for the subjective phenomenological effects of psychedelics has typically been based on LSD, with anything that is considered "psychedelic" evidently being compared to it and its specific effects.[112]
During a speech on his 100th birthday, the inventor of LSD, Albert Hofmann said of the drug: "It gave me an inner joy, an open mindedness, a gratefulness, open eyes and an internal sensitivity for the miracles of creation... I think that in human evolution it has never been as necessary to have this substance LSD. It is just a tool to turn us into what we are supposed to be."[113] With certain psychedelics and experiences, a user may also experience an "afterglow" of improved mood or perceived mental state for days or even weeks after ingestion in some cases.[114][115] In 1898, the English writer and intellectual Havelock Ellis reported a heightened perceptual sensitivity to "the more delicate phenomena of light and shade and color" for a prolonged period of time after his exposure to mescaline.[116] Good trips are reportedly deeply pleasurable, and typically involve intense joy or euphoria, a greater appreciation for life, reduced anxiety, a sense of spiritual enlightenment, and a sense of belonging or interconnectedness with the universe.[117][118] Negative experiences, colloquially known as "bad trips," evoke an array of dark emotions, such as irrational fear, anxiety, panic, paranoia, dread, distrustfulness, hopelessness, and even suicidal ideation.[119] While it is impossible to predict when a bad trip will occur, one's mood, surroundings, sleep, hydration, social setting, and other factors can be controlled (colloquially referred to as "set and setting") to minimize the risk of a bad trip.[120][121] The concept of "set and setting" also generally appears to be more applicable to psychedelics than to other types of hallucinogens such as deliriants, hypnotics and dissociative anesthetics.[122]
Classic psychedelics are considered to be those found in nature like psilocybin, DMT, mescaline, and LSD which is derived from naturally occurring ergotamine, and non-classic psychedelics are considered to be newer analogs and derivatives of pharmacophore lysergamides, tryptamine, and phenethylamine structures like 2C-B. Many of these psychedelics cause remarkably similar effects, despite their different chemical structure. However, many users report that the three major families have subjectively different qualities in the "feel" of the experience, which are difficult to describe. Some compounds, such as 2C-B, have extremely tight "dose curves", meaning the difference in dose between a non-event and an overwhelming disconnection from reality can be very slight. There can also be very substantial differences between the drugs; for instance, 5-MeO-DMT rarely produces the visual effects typical of other psychedelics.[11]
Potential adverse effects
[edit]Despite the contrary perception of much of the public, psychedelic drugs are not addictive and are physiologically safe.[18][19][11] As of 2016, there have been no known deaths due to overdose of LSD, psilocybin, or mescaline.[11]
Risks do exist during an unsupervised psychedelic experience, however; Ira Byock wrote in 2018 in the Journal of Palliative Medicine that psilocybin is safe when administered to a properly screened patient and supervised by a qualified professional with appropriate set and setting. However, he called for an "abundance of caution" because in the absence of these conditions a range of negative reactions is possible, including "fear, a prolonged sense of dread, or full panic." He notes that driving or even walking in public can be dangerous during a psychedelic experience because of impaired hand-eye coordination and fine motor control.[123] In some cases, individuals taking psychedelics have performed dangerous or fatal acts because they believed they possessed superhuman powers.[11]
Psilocybin-induced states of mind share features with states experienced in psychosis, and while a causal relationship between psilocybin and the onset of psychosis has not been established as of 2011, researchers have called for investigation of the relationship.[124] Many of the persistent negative perceptions of psychological risks are unsupported by the currently available scientific evidence, with the majority of reported adverse effects not being observed in a regulated and/or medical context.[125] A population study on associations between psychedelic use and mental illness published in 2013 found no evidence that psychedelic use was associated with increased prevalence of any mental illness.[126] In any case, induction of psychosis has been associated with psychedelics in small percentages of individuals, and the rates appear to be higher in people with schizophrenia.[127]
Using psychedelics poses certain risks of re-experiencing of the drug's effects, including flashbacks and hallucinogen persisting perception disorder (HPPD).[124] These non-psychotic effects are poorly studied, but the permanent symptoms (also called "endless trip") are considered to be rare.[128]
Serotonin syndrome can be caused by combining psychedelics with other serotonergic drugs, including certain antidepressants, opioids, CNS stimulants (e.g. MDMA), 5-HT1 agonists (e.g. triptans), herbs and others.[129][130][131][132]
Serotonergic psychedelics are agonists not only of the serotonin 5-HT2A receptor but also of the serotonin 5-HT2B receptor and other serotonin receptors.[133][134] A potential risk of frequent repeated use of serotonergic psychedelics is cardiac fibrosis and valvulopathy caused by 5-HT2B receptor activation.[133][134] However, single high doses or widely spaced doses (e.g., months) are widely thought to be safe and concerns about cardiac toxicity apply more to chronic psychedelic microdosing or very frequent use (e.g., weekly).[133][134] Selective 5-HT2A receptor agonists that do not activate the 5-HT2B receptor or other serotonin receptors, such as 25CN-NBOH, DMBMPP, and LPH-5, have been developed and are being studied.[135][81][136] Selective 5-HT2A receptor agonists are expected to avoid the cardiac risks of 5-HT2B receptor activation.[136]
Potential therapeutic effects
[edit]
Psychedelic substances which may have therapeutic uses include psilocybin, LSD, and mescaline.[23] During the 1950s and 1960s, lack of informed consent in some scientific trials on psychedelics led to significant, long-lasting harm to some participants.[23] Since then, research regarding the effectiveness of psychedelic therapy has been conducted under strict ethical guidelines, with fully informed consent and a pre-screening to avoid people with psychosis taking part.[23] Although the history behind these substances has hindered research into their potential medicinal value, scientists are now able to conduct studies and renew research that was halted in the 1970s. Some research has shown that these substances have helped people with such mental disorders as obsessive-compulsive disorder (OCD), post-traumatic stress disorder (PTSD), alcoholism, depression, and cluster headaches.[17]
It has long been known that psychedelics promote neurite growth and neuroplasticity and are potent psychoplastogens.[137][138][139] There is evidence that psychedelics induce molecular and cellular adaptations related to neuroplasticity and that these could potentially underlie therapeutic benefits.[140][141] Psychedelics have also been shown to have potent anti-inflammatory activity and therapeutic effects in animal models of inflammatory diseases including asthma,[142] and cardiovascular disease and diabetes.[143]
Surrounding culture
[edit]
Psychedelic culture includes manifestations such as psychedelic music,[144] psychedelic art,[145] psychedelic literature,[146] psychedelic film,[147] and psychedelic festivals.[148] Examples of psychedelic music would be rock bands like the Grateful Dead, Jefferson Airplane and The Beatles. Many psychedelic bands and elements of the psychedelic subculture originated in San Francisco during the mid to late 1960s.[149]
Legal status
[edit]Many psychedelics are classified under Schedule I of the United Nations Convention on Psychotropic Substances of 1971 as drugs with the greatest potential to cause harm and no acceptable medical uses.[150] In addition, many countries have analogue laws; for example, in the United States, the Federal Analogue Act of 1986 automatically forbids any drugs sharing similar chemical structures or chemical formulas to prohibited substances if sold for human consumption.[151]
In July 2022, though, under the United States Food and Drug Administration, the drug psilocybin was on track to be approved of as a treatment for depression, and MDMA as a treatment for post-traumatic stress disorder.[152]
U.S. states such as Oregon and Colorado have also instituted decriminalization and legalization measures for accessing psychedelics[153] and states like New Hampshire are attempting to do the same.[154] J.D. Tuccille argues that increasing rates of use of psychedelics in defiance of the law are likely to result in more widespread legalization and decriminalization of access to the substances in the United States (as has happened with alcohol and cannabis).[155]
See also
[edit]- Aztec use of entheogens
- Bwiti
- Cognitive liberty
- Concord Prison Experiment
- Designer Drugs
- Dissociative drug
- Deliriant
- Drug harmfulness
- Entheogenic drugs and the archaeological record
- Entheogenics and the Maya
- Hallucinogenic fish
- Hallucinogenic plants in Chinese herbals
- Hamilton's Pharmacopeia
- History of lysergic acid diethylamide
- Ibogaine
- List of designer drugs
- List of psychedelic drugs
- List of psychedelic plants
- Marsh Chapel Experiment
- Morning glory
- Mystical psychosis
- PiHKAL
- Psychedelia – Film about the history of psychedelic drugs
- Research chemical
- Serotonergic cell groups
- Serotonin syndrome
- Tabernanthe iboga
- TiHKAL
- Trip killer
Categories
[edit]Notes
[edit]- ^ The Food and Drug Administration describes the designation of breakthrough therapy as "a process designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s)."[66]
References
[edit]- ^ "Peyote San Pedro Cactus – Shamanic Sacraments". D.M.Taylor.
- ^ a b c Aghajanian, G (August 1999). "Serotonin and Hallucinogens". Neuropsychopharmacology. 21 (2): 16S–23S. doi:10.1016/S0893-133X(98)00135-3. PMID 10432484.
- ^ a b c Millière R, Carhart-Harris RL, Roseman L, Trautwein FM, Berkovich-Ohana A (2018). "Psychedelics, Meditation, and Self-Consciousness". Frontiers in Psychology. 9: 1475. doi:10.3389/fpsyg.2018.01475. PMC 6137697. PMID 30245648.
- ^ a b McClure-Begley TD, Roth BL (2022). "The promises and perils of psychedelic pharmacology for psychiatry". Nature Reviews Drug Discovery. 21 (6): 463–473. doi:10.1038/s41573-022-00421-7. PMID 35301459. S2CID 247521633. Retrieved 2024-02-08.
- ^ a b c d Timmermann C, Roseman L, Williams L, Erritzoe D, Martial C, Cassol H, et al. (2018). "DMT Models the Near-Death Experience". Frontiers in Psychology. 9: 1424. doi:10.3389/fpsyg.2018.01424. PMC 6107838. PMID 30174629.
- ^ a b R. R. Griffiths, W. A. Richards, U. McCann, R. Jesse (7 July 2006). "Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance". Psychopharmacology. 187 (3): 268–283. doi:10.1007/s00213-006-0457-5. PMID 16826400. S2CID 7845214.
- ^ McKenna, Terence (1992). Food of the Gods: The Search for the Original Tree of Knowledge A Radical History of Plants, Drugs, and Human Evolution
- ^ W. Davis (1996), One River: Explorations and Discoveries in the Amazon Rain Forest. New York, Simon and Schuster, Inc. p. 120.
- ^ a b "Crystal Structure of LSD and 5-HT2AR Part 2: Binding Details and Future Psychedelic Research Paths". Psychedelic Science Review. 2020-10-05. Retrieved 2021-02-12.
- ^ a b c Nichols DE (2018). "Chemistry and Structure–Activity Relationships of Psychedelics". In Halberstadt AL, Vollenweider FX, Nichols DE (eds.). Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. Vol. 36. Berlin: Springer. pp. 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55880-5. PMID 28401524.
- ^ a b c d e f g h Nichols DE (2016). "Psychedelics". Pharmacological Reviews. 68 (2): 264–355. doi:10.1124/pr.115.011478. ISSN 0031-6997. PMC 4813425. PMID 26841800.
- ^ Siegel GJ (11 November 2005). Basic neurochemistry: Molecular, cellular and medical aspects (7th ed.). Amsterdam: Elsevier Science. ISBN 978-0-08-047207-2. OCLC 123438340.
- ^ Smigielski L, Scheidegger M, Kometer M, Vollenweider FX (August 2019). "Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects". NeuroImage. 196: 207–215. doi:10.1016/j.neuroimage.2019.04.009. PMID 30965131. S2CID 102487343.
- ^ a b Letheby C, Gerrans P (2017). "Self unbound: ego dissolution in psychedelic experience". Neuroscience of Consciousness. 3 (1): nix016. doi:10.1093/nc/nix016. PMC 6007152. PMID 30042848.
The connection with findings about PCC deactivation in 'effortless awareness' meditation is obvious, and bolstered by the finding that acute ayahuasca intoxication increases mindfulness-related capacities.
- ^ Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H (2011-12-13). "Meditation experience is associated with differences in default mode network activity and connectivity". Proceedings of the National Academy of Sciences. 108 (50): 20254–20259. Bibcode:2011PNAS..10820254B. doi:10.1073/pnas.1112029108. PMC 3250176. PMID 22114193.
- ^ a b Krebs, Teri S, Johansen, Pål-Ørjan (28 March 2013). "Over 30 million psychedelic users in the United States". F1000Research. 2: 98. doi:10.12688/f1000research.2-98.v1. PMC 3917651. PMID 24627778.
- ^ a b c d Garcia-Romeu, Albert, Kersgaard, Brennan, Addy, Peter H. (August 2016). "Clinical applications of hallucinogens: A review". Experimental and Clinical Psychopharmacology. 24 (4): 229–268. doi:10.1037/pha0000084. PMC 5001686. PMID 27454674.
- ^ a b Le Dain G (1971). The Non-medical Use of Drugs: Interim Report of the Canadian Government's Commission of Inquiry. p. 106.
Physical dependence does not develop to LSD
- ^ a b Lüscher, Christian, Ungless, Mark A. (14 November 2006). "The Mechanistic Classification of Addictive Drugs". PLOS Medicine. 3 (11): e437. doi:10.1371/journal.pmed.0030437. PMC 1635740. PMID 17105338.
- ^ Rich Haridy (24 October 2018). "Psychedelic psilocybin therapy for depression granted Breakthrough Therapy status by FDA". newatlas.com. Retrieved 2019-09-27.
- ^ a b c d Bender D, Hellerstein DJ (2022). "Assessing the risk–benefit profile of classical psychedelics: a clinical review of second-wave psychedelic research". Psychopharmacology. 239 (6): 1907–1932. doi:10.1007/s00213-021-06049-6. PMID 35022823. S2CID 245906937.
- ^ a b c Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, et al. (May 2020). "Psychedelics and Psychedelic-Assisted Psychotherapy". The American Journal of Psychiatry. 177 (5): 391–410. doi:10.1176/appi.ajp.2019.19010035. PMID 32098487. S2CID 211524704.
- ^ a b c d Tupper KW, Wood E, Yensen R, Johnson MW (2015-10-06). "Psychedelic medicine: a re-emerging therapeutic paradigm". CMAJ: Canadian Medical Association Journal. 187 (14): 1054–1059. doi:10.1503/cmaj.141124. ISSN 0820-3946. PMC 4592297. PMID 26350908.
- ^ Orth T (July 28, 2022). "One in four Americans say they've tried at least one psychedelic drug". YouGov.
- ^ Tanne JH (2004-03-20). "Humphry Osmond". BMJ: British Medical Journal. 328 (7441): 713. ISSN 0959-8138. PMC 381240.
- ^ Tanne JH (2004). "Humphrey Osmond". BMJ. 328 (7441): 713. doi:10.1136/bmj.328.7441.713. PMC 381240.
- ^ Oxford English Dictionary, 3rd edition, September 2007, s.v., Etymology
- ^ A. Weil, W. Rosen. (1993), From Chocolate To Morphine: Everything You Need To Know About Mind-Altering Drugs. New York, Houghton Mifflin Company. p. 93
- ^ W. Davis (1996), "One River: Explorations and Discoveries in the Amazon Rain Forest". New York, Simon and Schuster, Inc. p. 120.
- ^ iia700700.us.archive.org
- ^ a b c d e Nichols, David E. (2004). "Hallucinogens". Pharmacology & Therapeutics. 101 (2): 131–81. doi:10.1016/j.pharmthera.2003.11.002. PMID 14761703.
- ^ Carhart-Harris R, Guy G (2017). "The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future". Neuropsychopharmacology. 42 (11): 2105–2113. doi:10.1038/npp.2017.84. PMC 5603818. PMID 28443617.
- ^ DiVito AJ, Leger RF (2020). "Psychedelics as an emerging novel intervention in the treatment of substance use disorder: a review". Molecular Biology Reports. 47 (12): 9791–9799. doi:10.1007/s11033-020-06009-x. PMID 33231817. S2CID 227157734.
- ^ a b c Marks M, Cohen IG (October 2021). "Psychedelic therapy: a roadmap for wider acceptance and utilization". Nature Medicine. 27 (10): 1669–1671. doi:10.1038/s41591-021-01530-3. PMID 34608331. S2CID 238355863.
- ^ Siegel AN, Meshkat S, Benitah K, Lipstiz O, Gill H, Lui LM, et al. (2021). "Registered clinical studies investigating psychedelic drugs for psychiatric disorders". Journal of Psychiatric Research. 139: 71–81. doi:10.1016/j.jpsychires.2021.05.019. PMID 34048997. S2CID 235242044.
- ^ Andersen KA, Carhart-Harris R, Nutt DJ, Erritzoe D (2020). "Therapeutic effects of classic serotonergic psychedelics: A systematic review of modern-era clinical studies". Acta Psychiatrica Scandinavica. 143 (2): 101–118. doi:10.1111/acps.13249. PMID 33125716. S2CID 226217912.
- ^ Malcolm B, Thomas K (2022). "Serotonin toxicity of serotonergic psychedelics". Psychopharmacology. 239 (6): 1881–1891. doi:10.1007/s00213-021-05876-x. PMID 34251464. S2CID 235796130.
- ^ a b Shulgin AT (1991). Pihkal : a chemical love story. Ann Shulgin. Berkeley, CA: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ (July 2003). "Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion, and Pharmacokinetics". Journal of Pharmacology and Experimental Therapeutics. 306 (1): 73–83. doi:10.1124/jpet.103.049882. ISSN 0022-3565. PMID 12660312. S2CID 6147566.
- ^ Haroz R, Greenberg MI (November 2005). "Emerging drugs of abuse". The Medical Clinics of North America. 89 (6): 1259–1276. doi:10.1016/j.mcna.2005.06.008. ISSN 0025-7125. PMID 16227062.
- ^ Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R (1994-02-01). "Dose-Response Study of N,N-Dimethyltryptamine in Humans: II. Subjective Effects and Preliminary Results of a New Rating Scale". Archives of General Psychiatry. 51 (2): 98–108. doi:10.1001/archpsyc.1994.03950020022002. ISSN 0003-990X. PMID 8297217.
- ^ Davis AK, Clifton JM, Weaver EG, Hurwitz ES, Johnson MW, Griffiths RR (September 2020). "Survey of entity encounter experiences occasioned by inhaled N,N -dimethyltryptamine: Phenomenology, interpretation, and enduring effects". Journal of Psychopharmacology. 34 (9): 1008–1020. doi:10.1177/0269881120916143. ISSN 0269-8811. PMID 32345112.
- ^ Creese I, Burt DR, Snyder SH (1975-12-01). "The dopamine receptor: Differential binding of d-LSD and related agents to agonist and antagonist states". Life Sciences. 17 (11): 1715–1719. doi:10.1016/0024-3205(75)90118-6. ISSN 0024-3205. PMID 1207384.
- ^ Passie T, Seifert J, Schneider U, Emrich HM (October 2002). "The pharmacology of psilocybin". Addiction Biology. 7 (4): 357–364. doi:10.1080/1355621021000005937. PMID 14578010. S2CID 12656091.
- ^ Freye E (2009). Pharmacology and abuse of cocaine, amphetamines, ecstasy and related designer drugs : a comprehensive review on their mode of action, treatment of abuse and intoxication. Dordrecht: Springer. ISBN 978-90-481-2448-0. OCLC 489218895.
- ^ Bohn A, Kiggen MH, Uthaug MV, van Oorsouw KI, Ramaekers JG, van Schie HT (2022-12-05). "Altered States of Consciousness During Ceremonial San Pedro Use". The International Journal for the Psychology of Religion. 33 (4): 309–331. doi:10.1080/10508619.2022.2139502. hdl:2066/285968. ISSN 1050-8619.
- ^ Griffiths RR, Richards WA, McCann U, Jesse R (August 2006). "Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance". Psychopharmacology. 187 (3): 268–283. doi:10.1007/s00213-006-0457-5. ISSN 0033-3158. PMID 16826400. S2CID 7845214.
- ^ a b "4-HO-MiPT". Psychedelic Science Review. 2020-01-06. Retrieved 2022-07-06.
- ^ Carlini EA, Maia LO (2020). "Plant and Fungal Hallucinogens as Toxic and Therapeutic Agents". Plant Toxins. Toxinology. Springer Netherlands. pp. 1–44. doi:10.1007/978-94-007-6728-7_6-2. ISBN 978-94-007-6728-7. S2CID 239438352. Retrieved 23 February 2022.
- ^ "History of Psychedelics: How the Mazatec Tribe Brought Entheogens to the World". Psychedelic Times. 28 October 2015. Retrieved 23 February 2022.
- ^ Ismael Eduardo Apud Peláez. (2020). Ayahuasca: Between Cognition and Culture. Publicacions Universitat Rovira i Virgili. ISBN 978-84-8424-834-7. OCLC 1229544084.
- ^ Prince MA, O'Donnell MB, Stanley LR, Swaim RC (May 2019). "Examination of Recreational and Spiritual Peyote Use Among American Indian Youth". Journal of Studies on Alcohol and Drugs. 80 (3): 366–370. doi:10.15288/jsad.2019.80.366. PMC 6614926. PMID 31250802.
- ^ Bussmann RW, Sharon D (2006). "Traditional medicinal plant use in Northern Peru: tracking two thousand years of healing culture". J Ethnobiol Ethnomed. 2 (1): 47. doi:10.1186/1746-4269-2-47. PMC 1637095. PMID 17090303.
- ^ Socha DM, Sykutera M, Orefici G (2022-12-01). "Use of psychoactive and stimulant plants on the south coast of Peru from the Early Intermediate to Late Intermediate Period". Journal of Archaeological Science. 148: 105688. Bibcode:2022JArSc.148j5688S. doi:10.1016/j.jas.2022.105688. ISSN 0305-4403. S2CID 252954052.
- ^ Larco L (2008). "Archivo Arquidiocesano de Trujillo Sección Idolatrías. (Años 1768–1771)". Más allá de los encantos – Documentos sobre extirpación de idolatrías, Trujillo. Travaux de l'IFEA. Lima: IFEA Instituto Francés de Estudios Andinos, Fondo Editorial de la Universidad Nacional Mayor de San Marcos. pp. 67–87. ISBN 978-2-8218-4453-7. Retrieved April 9, 2020.
- ^ Anderson EF (2001). The Cactus Family. Pentland, Oregon: Timber Press. ISBN 978-0-88192-498-5. pp. 45–49.
- ^ "Declaran Patrimonio Cultural de la Nación a los conocimientos, saberes y usos del cactus San Pedro". elperuano.pe (in Spanish). 2022-11-17. Retrieved 2022-12-10.
- ^ a b "Psychedelics Weren't As Common in Ancient Cultures As We Think". Vice Media. Vice. December 10, 2020. Retrieved January 14, 2023.
- ^ Nutt D, Spriggs M, Erritzoe D (2023-02-01). "Psychedelics therapeutics: What we know, what we think, and what we need to research". Neuropharmacology. 223: 109257. doi:10.1016/j.neuropharm.2022.109257. ISSN 0028-3908. PMID 36179919.
- ^ Pilecki B, Luoma JB, Bathje GJ, Rhea J, Narloch VF (2021). "Ethical and legal issues in psychedelic harm reduction and integration therapy". Harm Reduction Journal. 18 (1): 40. doi:10.1186/s12954-021-00489-1. PMC 8028769. PMID 33827588.
- ^ Nutt D, Erritzoe D, Carhart-Harris R (2020). "Psychedelic Psychiatry's Brave New World". Cell. 181 (1): 24–28. doi:10.1016/j.cell.2020.03.020. PMID 32243793. S2CID 214753833.
- ^ a b Johnson MW, Richards WA, Griffiths RR (2008). "Human hallucinogen research: guidelines for safety". Journal of Psychopharmacology. 22 (6): 603–620. doi:10.1177/0269881108093587. PMC 3056407. PMID 18593734.
- ^ a b Garcia-Romeu A, Richards WA (2018). "Current perspectives on psychedelic therapy: use of serotonergic hallucinogens in clinical interventions". International Review of Psychiatry. 30 (4): 291–316. doi:10.1080/09540261.2018.1486289. ISSN 0954-0261. PMID 30422079. S2CID 53291327.
- ^ Romeo B, Karila L, Martelli C, Benyamina A (2020). "Efficacy of psychedelic treatments on depressive symptoms: A meta-analysis". Journal of Psychopharmacology. 34 (10): 1079–1085. doi:10.1177/0269881120919957. PMID 32448048. S2CID 218873949.
- ^ Schimmel N, Breeksema JJ, Smith-Apeldoorn SY, Veraart J, van den Brink W, Schoevers RA (2022). "Psychedelics for the treatment of depression, anxiety, and existential distress in patients with a terminal illness: a systematic review". Psychopharmacology. 239 (15–33): 15–33. doi:10.1007/s00213-021-06027-y. PMID 34812901. S2CID 244490236.
- ^ "Breakthrough Therapy". United States Food and Drug Administration. 1 April 2018. Archived from the original on 1 March 2022. Retrieved 27 March 2022.
- ^ Krebs, Teri S., Johansen, Pål-Ørjan (August 19, 2013). "Psychedelics and Mental Health: A Population Study". PLOS ONE. 8 (8): e63972. Bibcode:2013PLoSO...863972K. doi:10.1371/journal.pone.0063972. PMC 3747247. PMID 23976938.
- ^ Goldman M (2024-06-27). "Mushrooms are the most commonly used psychedelic, study finds". Axios. Retrieved 2024-06-28.
- ^ Fadiman J (2016-01-01). "Microdose research: without approvals, control groups, double blinds, staff or funding". Psychedelic Press. XV.
- ^ Brodwin E (30 January 2017). "The truth about 'microdosing,' which involves taking tiny amounts of psychedelics like LSD". Business Insider. Retrieved 19 April 2017.
- ^ Dahl H (7 July 2015). "A Brief History of LSD in the Twenty-First Century". Psychedelic Press UK. Retrieved 19 April 2017.
- ^ Webb M, Copes H, Hendricks PS (August 2019). "Narrative identity, rationality, and microdosing classic psychedelics". The International Journal on Drug Policy. 70: 33–39. doi:10.1016/j.drugpo.2019.04.013. PMID 31071597. S2CID 149445841.
- ^ Chemistry Uo, Prague T. "Recognizing signatures of psilocybin microdosing in natural language". medicalxpress.com. Retrieved 2022-10-03.
- ^ Sanz C, Cavanna F, Muller S, de la Fuente L, Zamberlan F, Palmucci M, et al. (2022-09-01). "Natural language signatures of psilocybin microdosing". Psychopharmacology. 239 (9): 2841–2852. doi:10.1007/s00213-022-06170-0. ISSN 1432-2072. PMID 35676541. S2CID 247067976.
- ^ Dolan EW (2023-06-02). "Neuroscience research sheds light on how LSD alters the brain's "gatekeeper"". Psypost - Psychology News. Retrieved 2023-06-02.
- ^ Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. (2018). "Psychedelics promote structural and functional neural plasticity". Cell Reports. 23 (11): 3170–3182. doi:10.1016/j.celrep.2018.05.022. PMC 6082376. PMID 29898390.
- ^ Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, et al. (2023-02-17). "Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors". Science. 379 (6633): 700–706. Bibcode:2023Sci...379..700V. doi:10.1126/science.adf0435. ISSN 0036-8075. PMC 10108900. PMID 36795823.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x Halberstadt AL (January 2015). "Recent advances in the neuropsychopharmacology of serotonergic hallucinogens". Behav Brain Res. 277: 99–120. doi:10.1016/j.bbr.2014.07.016. PMID 25036425.
- ^ a b c d e f g h i j k l Kwan AC, Olson DE, Preller KH, Roth BL (November 2022). "The neural basis of psychedelic action". Nat Neurosci. 25 (11): 1407–1419. doi:10.1038/s41593-022-01177-4. PMID 36280799.
- ^ Halberstadt AL, Nichols DE (2020). "Serotonin and serotonin receptors in hallucinogen action". Handbook of Behavioral Neuroscience. Vol. 31. Elsevier. pp. 843–863. doi:10.1016/b978-0-444-64125-0.00043-8. ISBN 978-0-444-64125-0. ISSN 1569-7339.
- ^ a b c d e f Duan W, Cao D, Wang S, Cheng J (January 2024). "Serotonin 2A Receptor (5-HT2AR) Agonists: Psychedelics and Non-Hallucinogenic Analogues as Emerging Antidepressants". Chem Rev. 124 (1): 124–163. doi:10.1021/acs.chemrev.3c00375. PMID 38033123.
- ^ Roth BL, Gumpper RH (May 2023). "Psychedelics as Transformative Therapeutics". Am J Psychiatry. 180 (5): 340–347. doi:10.1176/appi.ajp.20230172. PMID 37122272.
- ^ Kozlenkov A, González-Maeso J (2013). "Animal Models and Hallucinogenic Drugs". The Neuroscience of Hallucinations. New York, NY: Springer New York. p. 253–277. doi:10.1007/978-1-4614-4121-2_14. ISBN 978-1-4614-4120-5.
- ^ a b c Glennon RA, Dukat M (June 2024). "1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI): From an Obscure to Pivotal Member of the DOX Family of Serotonergic Psychedelic Agents - A Review". ACS Pharmacology & Translational Science. 7 (6): 1722–1745. doi:10.1021/acsptsci.4c00157. PMID 38898956.
Although the specific signaling cascades mediating the HTR have not been conclusively identified, Gq and β-arrestin2 have been implicated. Recent studies with different existing and novel agents, including DOI, found that the HTR was correlated with Gq efficacy but not with β-arrestin2 recruitment.114
- ^ a b c d Barksdale BR, Doss MK, Fonzo GA, Nemeroff CB (March 2024). "The mechanistic divide in psychedelic neuroscience: An unbridgeable gap?". Neurotherapeutics. 21 (2): e00322. doi:10.1016/j.neurot.2024.e00322. PMID 38278658.
- ^ a b c Gumpper RH, Nichols DE (October 2024). "Chemistry/structural biology of psychedelic drugs and their receptor(s)". Br J Pharmacol. doi:10.1111/bph.17361. PMID 39354889.
- ^ Chisamore N, Kaczmarek E, Le GH, Wong S, Orsini DK, Mansur R, et al. (26 April 2024). "Neurobiology of the Antidepressant Effects of Serotonergic Psychedelics: A Narrative Review". Current Treatment Options in Psychiatry. 11 (2). Springer Science and Business Media LLC: 90–105. doi:10.1007/s40501-024-00319-8. ISSN 2196-3061.
- ^ a b c d e Wallach J, Cao AB, Calkins MM, Heim AJ, Lanham JK, Bonniwell EM, et al. (December 2023). "Identification of 5-HT2A receptor signaling pathways associated with psychedelic potential". Nat Commun. 14 (1): 8221. doi:10.1038/s41467-023-44016-1. PMID 38102107.
- ^ Glatfelter GC, Pottie E, Partilla JS, Stove CP, Baumann MH (March 2024). "Comparative Pharmacological Effects of Lisuride and Lysergic Acid Diethylamide Revisited". ACS Pharmacol Transl Sci. 7 (3): 641–653. doi:10.1021/acsptsci.3c00192. PMID 38481684.
- ^ Lewis V, Bonniwell EM, Lanham JK, Ghaffari A, Sheshbaradaran H, Cao AB, et al. (March 2023). "A non-hallucinogenic LSD analog with therapeutic potential for mood disorders". Cell Rep. 42 (3): 112203. doi:10.1016/j.celrep.2023.112203. PMID 36884348.
- ^ Cunningham MJ, Bock HA, Serrano IC, Bechand B, Vidyadhara DJ, Bonniwell EM, et al. (January 2023). "Pharmacological Mechanism of the Non-hallucinogenic 5-HT2A Agonist Ariadne and Analogs". ACS Chem Neurosci. 14 (1): 119–135. doi:10.1021/acschemneuro.2c00597. PMID 36521179.
- ^ Davis R, Dutheil SS, Zhang L, Lehmann E, Awadallah N, Yao W, et al. (December 2023). "ACNP 62nd Annual Meeting: Poster Abstracts P251 - P500: P358. Discovery and Characterization of ITI-1549, a Novel Non-Hallucinogenic Psychedelic for the Treatment of Neuropsychiatric Disorders". Neuropsychopharmacology. 48 (Suppl 1): 211–354 (272–273). doi:10.1038/s41386-023-01756-4. PMC 10729596. PMID 38040810.
- ^ Aqil M, Roseman L (February 2023). "More than meets the eye: The role of sensory dimensions in psychedelic brain dynamics, experience, and therapeutics". Neuropharmacology. 223: 109300. doi:10.1016/j.neuropharm.2022.109300. PMID 36334767.
- ^ Császár-Nagy N, Kapócs G, Bókkon I (July 2019). "Classic psychedelics: the special role of the visual system". Rev Neurosci. 30 (6): 651–669. doi:10.1515/revneuro-2018-0092. PMID 30939118.
- ^ Bressloff PC, Cowan JD, Golubitsky M, Thomas PJ, Wiener MC (March 2002). "What geometric visual hallucinations tell us about the visual cortex". Neural Comput. 14 (3): 473–491. doi:10.1162/089976602317250861. PMID 11860679.
- ^ Cameron LP, Benetatos J, Lewis V, Bonniwell EM, Jaster AM, Moliner R, et al. (November 2023). "Beyond the 5-HT2A Receptor: Classic and Nonclassic Targets in Psychedelic Drug Action". J Neurosci. 43 (45): 7472–7482. doi:10.1523/JNEUROSCI.1384-23.2023. PMID 37940583.
- ^ Wölfel R, Graefe KH (February 1992). "Evidence for various tryptamines and related compounds acting as substrates of the platelet 5-hydroxytryptamine transporter". Naunyn-Schmiedeberg's Archives of Pharmacology. 345 (2): 129–136. doi:10.1007/BF00165727. ISSN 0028-1298. PMID 1570019. S2CID 2984583.
- ^ Shimazu S, Miklya I (May 2004). "Pharmacological studies with endogenous enhancer substances: β-phenylethylamine, tryptamine, and their synthetic derivatives". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 28 (3): 421–427. doi:10.1016/j.pnpbp.2003.11.016. PMID 15093948. S2CID 37564231.
- ^ Blough BE, Landavazo A, Partilla JS, Baumann MH, Decker AM, Page KM, et al. (2014-06-12). "Hybrid Dopamine Uptake Blocker–Serotonin Releaser Ligands: A New Twist on Transporter-Focused Therapeutics". ACS Medicinal Chemistry Letters. 5 (6): 623–627. doi:10.1021/ml500113s. ISSN 1948-5875. PMC 4060932. PMID 24944732.
- ^ "4-HO-MET". The Drug Classroom. Retrieved 2022-07-31.
- ^ Shulgin, Alexander T. (Alexander Theodore) (1997). Tihkal : the continuation. Shulgin, Ann. (1st ed.). Berkeley, CA: Transform Press. ISBN 0-9630096-9-9. OCLC 38503252.
- ^ Miller GM (2010-12-16). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. ISSN 0022-3042. PMC 3005101. PMID 21073468.
- ^ Grandy G (2020-04-23). "Guest editorial". Gender in Management. 35 (3): 257–260. doi:10.1108/GM-05-2020-238. ISSN 1754-2413.
- ^ Little KY, Krolewski DM, Zhang L, Cassin BJ (January 2003). "Loss of Striatal Vesicular Monoamine Transporter Protein (VMAT2) in Human Cocaine Users". American Journal of Psychiatry. 160 (1): 47–55. doi:10.1176/appi.ajp.160.1.47. ISSN 0002-953X. PMID 12505801.
- ^ Brandt SD, Kavanagh PV, Westphal F, Stratford A, Odland AU, Klein AK, et al. (2020-04-20). "Return of the lysergamides. Part VI: Analytical and behavioural characterization of 1-cyclopropanoyl- d -lysergic acid diethylamide (1CP-LSD)" (PDF). Drug Testing and Analysis. 12 (6): 812–826. doi:10.1002/dta.2789. ISSN 1942-7603. PMC 9191646. PMID 32180350. S2CID 212738912.
- ^ Grumann C, Henkel K, Brandt SD, Stratford A, Passie T, Auwärter V (2020). "Pharmacokinetics and subjective effects of 1P-LSD in humans after oral and intravenous administration". Drug Testing and Analysis. 12 (8): 1144–1153. doi:10.1002/dta.2821. ISSN 1942-7611. PMID 32415750.
- ^ Preller KH, Vollenweider FX (2016). "Phenomenology, Structure, and Dynamic of Psychedelic States". In Adam L. Halberstadt, Franz X. Vollenweider, David E. Nichols (eds.). Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. Vol. 36. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 221–256. doi:10.1007/7854_2016_459. ISBN 978-3-662-55878-2. PMID 28025814.
- ^ Swanson LR (2018-03-02). "Unifying Theories of Psychedelic Drug Effects". Frontiers in Pharmacology. 9: 172. doi:10.3389/fphar.2018.00172. ISSN 1663-9812. PMC 5853825. PMID 29568270.
- ^ Luke, David (28 November 2013). "Rock Art or Rorschach: Is there More to Entoptics than Meets the Eye?". Time and Mind. 3 (1): 9–28. doi:10.2752/175169710x12549020810371. S2CID 144948636.
- ^ Berry MD (July 2004). "Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators". Journal of Neurochemistry. 90 (2): 257–271. doi:10.1111/j.1471-4159.2004.02501.x. ISSN 0022-3042. PMID 15228583. S2CID 12126296.
- ^ Luna LE (1984). "The concept of plants as teachers among four mestizo shamans of Iquitos, northeastern Peru" (PDF). Journal of Ethnopharmacology. 11 (2): 135–156. doi:10.1016/0378-8741(84)90036-9. PMID 6492831. Retrieved 10 July 2020.
- ^ Nichols DE (April 2016). "Psychedelics". Pharmacological Reviews. 68 (2): 264–355. doi:10.1124/pr.115.011478. PMC 4813425. PMID 26841800.
- ^ "LSD: The Geek's Wonder Drug?". Wired. 16 January 2006. Retrieved 29 April 2008.
- ^ Majić T, Schmidt TT, Gallinat J (March 2015). "Peak experiences and the afterglow phenomenon: when and how do therapeutic effects of hallucinogens depend on psychedelic experiences?". Journal of Psychopharmacology. 29 (3): 241–53. doi:10.1177/0269881114568040. PMID 25670401. S2CID 16483172.
- ^ Evens R, Schmidt ME, Majić T, Schmidt TT (2023-05-29). "The psychedelic afterglow phenomenon: a systematic review of subacute effects of classic serotonergic psychedelics". Therapeutic Advances in Psychopharmacology. 13: 20451253231172254. doi:10.1177/20451253231172254. ISSN 2045-1253. PMC 10240558. PMID 37284524.
- ^ Hanson D (29 April 2013). "When the Trip Never Ends". Dana Foundation.
- ^ Honig D. "Frequently Asked Questions". Erowid. Archived from the original on 12 February 2016.
- ^ McGlothlin W, Cohen S, McGlothlin MS (November 1967). "Long lasting effects of LSD on normals" (PDF). Archives of General Psychiatry. 17 (5): 521–32. doi:10.1001/archpsyc.1967.01730290009002. PMID 6054248. Archived from the original (PDF) on April 30, 2011.
- ^ Canadian government (1996). "Controlled Drugs and Substances Act". Justice Laws. Canadian Department of Justice. Archived from the original on December 15, 2013. Retrieved December 15, 2013.
- ^ Rogge T (May 21, 2014), Substance use – LSD, MedlinePlus, U.S. National Library of Medicine, archived from the original on July 28, 2016, retrieved July 14, 2016
- ^ CESAR (29 October 2013), LSD, Center for Substance Abuse Research, University of Maryland, archived from the original on July 15, 2016, retrieved 14 July 2016
- ^ Garcia-Romeu A, Kersgaard B, Addy PH (August 2016). "Clinical applications of hallucinogens: A review". Experimental and Clinical Psychopharmacology. 24 (4): 229–268. doi:10.1037/pha0000084. PMC 5001686. PMID 27454674.
- ^ Byock I (2018). "Taking Psychedelics Seriously". Journal of Palliative Medicine. 21 (4): 417–421. doi:10.1089/jpm.2017.0684. PMC 5867510. PMID 29356590.
- ^ a b van Amsterdam J, Opperhuizen A, van den Brink W (2011). "Harm potential of magic mushroom use: A review". Regulatory Toxicology and Pharmacology. 59 (3): 423–429. doi:10.1016/j.yrtph.2011.01.006. PMID 21256914.
- ^ Schlag AK, Aday J, Salam I, Neill JC, Nutt DJ (2022-02-02). "Adverse effects of psychedelics: From anecdotes and misinformation to systematic science". Journal of Psychopharmacology. 36 (3): 258–272. doi:10.1177/02698811211069100. ISSN 0269-8811. PMC 8905125. PMID 35107059.
- ^ Krebs, Teri S., Johansen, Pål-Ørjan, Lu, Lin (19 August 2013). "Psychedelics and Mental Health: A Population Study". PLOS ONE. 8 (8): e63972. Bibcode:2013PLoSO...863972K. doi:10.1371/journal.pone.0063972. PMC 3747247. PMID 23976938.
- ^ Sabé M, Sulstarova A, Glangetas A, De Pieri M, Mallet L, Curtis L, et al. (November 2024). "Reconsidering evidence for psychedelic-induced psychosis: an overview of reviews, a systematic review, and meta-analysis of human studies". Mol Psychiatry. doi:10.1038/s41380-024-02800-5. PMID 39592825.
- ^ Baggott MJ, Coyle JR, Erowid E, Erowid F, Robertson LC (1 March 2011). "Abnormal visual experiences in individuals with histories of hallucinogen use: A web-based questionnaire". Drug and Alcohol Dependence. 114 (1): 61–67. doi:10.1016/j.drugalcdep.2010.09.006. PMID 21035275.
- ^ Bijl D (October 2004). "The serotonin syndrome". Neth J Med. 62 (9): 309–13. PMID 15635814.
Mechanisms of serotonergic drugs implicated in serotonin syndrome... Stimulation of serotonin receptors... LSD
- ^ "AMT". DrugWise.org.uk. 2016-01-03. Retrieved 2019-11-18.
- ^ Alpha-methyltryptamine (AMT) – Critical Review Report (PDF) (Report). World Health Organisation – Expert Committee on Drug Dependence (published 2014-06-20). 20 June 2014. Retrieved 2019-11-18.
- ^ Boyer EW, Shannon M (March 2005). "The serotonin syndrome" (PDF). The New England Journal of Medicine. 352 (11): 1112–20. doi:10.1056/NEJMra041867. PMID 15784664. Archived (PDF) from the original on 2013-06-18.
- ^ a b c Tagen M, Mantuani D, van Heerden L, Holstein A, Klumpers LE, Knowles R (September 2023). "The risk of chronic psychedelic and MDMA microdosing for valvular heart disease". J Psychopharmacol. 37 (9): 876–890. doi:10.1177/02698811231190865. PMID 37572027.
- ^ a b c Rouaud A, Calder AE, Hasler G (March 2024). "Microdosing psychedelics and the risk of cardiac fibrosis and valvulopathy: Comparison to known cardiotoxins". J Psychopharmacol. 38 (3): 217–224. doi:10.1177/02698811231225609. PMC 10944580. PMID 38214279.
- ^ Märcher Rørsted E, Jensen AA, Kristensen JL (November 2021). "25CN-NBOH: A Selective Agonist for in vitro and in vivo Investigations of the Serotonin 2A Receptor". ChemMedChem. 16 (21): 3263–3270. doi:10.1002/cmdc.202100395. PMID 34288515.
- ^ a b Peplow M (2024). "Next-generation psychedelics: should new agents skip the trip?". Nature Biotechnology. 42 (6): 827–830. doi:10.1038/s41587-024-02285-1. ISSN 1087-0156.
Another problem is that some classical psychedelics are also agonists of the 5-HT2B receptor, which is expressed in heart tissue and can cause long-term cardiac problems. Kristensen's company Lophora aims to solve that with its lead compound LPH-5, a phenylethylamine derivative with an extra molecular ring that makes it less flexible. LPH-5 has a 60-fold higher selectivity for 5-HT2A over 5-HT2B.
- ^ Jones K, Srivastave D, Allen J, Roth B, Penzes P (2009). "Psychedelics Promote Structural and Functional Neural Plasticity". Proc Natl Acad Sci U S A. 106 (46): 19575–19580. doi:10.1073/pnas.0905884106. PMC 2780750. PMID 19889983.
- ^ Yoshida H, Kanamaru C, Ohtani A, Senzaki K, Shiga T (2011). "Subtype specific roles of serotonin receptors in the spine formation of cortical neurons in vitro" (PDF). Neurosci Res. 71 (3): 311–314. doi:10.1016/j.neures.2011.07.1824. hdl:2241/114624. PMID 21802453. S2CID 14178672.
- ^ Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. (June 2018). "Psychedelics Promote Structural and Functional Neural Plasticity". Cell Reports. 23 (11): 3170–3182. doi:10.1016/j.celrep.2018.05.022. PMC 6082376. PMID 29898390.
- ^ de Vos CM, Mason NL, Kuypers KP (2021). "Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics". Frontiers in Psychiatry. 12: 1575. doi:10.3389/fpsyt.2021.724606. ISSN 1664-0640. PMC 8461007. PMID 34566723.
- ^ Calder AE, Hasler G (2022-09-19). "Towards an understanding of psychedelic-induced neuroplasticity". Neuropsychopharmacology. 48 (1): 104–112. doi:10.1038/s41386-022-01389-z. ISSN 1740-634X. PMC 9700802. PMID 36123427. S2CID 252381170.
- ^ Nau F, Miller J, Saravia J, Ahlert T, Yu B, Happel K, et al. (2015). "Serotonin 5-HT₂ receptor activation prevents allergic asthma in a mouse model". American Journal of Physiology. Lung Cellular and Molecular Physiology. 308 (2): 191–198. doi:10.1152/ajplung.00138.2013. PMC 4338939. PMID 25416380.
- ^ Flanagan T, Sebastian M, Battaglia D, Foster T, Maillet E, Nichols C (2019). "Activation of 5-HT2 Receptors Reduces Inflammation in Vascular Tissue and Cholesterol Levels in High-Fat Diet-Fed Apolipoprotein E Knockout Mice". Sci. Rep. 9 (1): 13444–198. Bibcode:2019NatSR...913444F. doi:10.1038/s41598-019-49987-0. PMC 6748996. PMID 31530895.
- ^ Hicks M (15 January 2000). Sixties Rock: Garage, Psychedelic, and Other Satisfactions. Chicago, IL: University of Illinois Press. pp. 63–64. ISBN 0-252-06915-3.
- ^ Krippner S (2017). "Ecstatic Landscapes: The Manifestation of Psychedelic Art". Journal of Humanistic Psychology. 57 (4): 415–435. doi:10.1177/0022167816671579. S2CID 151517152.
- ^ Dickins R (2013). "Preparing the Gaia connection: An ecological exposition of psychedelic literature 1954-1963". European Journal of Ecopsychology. 4: 9–18. CiteSeerX 10.1.1.854.6673. Retrieved 7 January 2021.
- ^ Gallagher M (2004). "Tripped Out: The Psychedelic Film and Masculinity". Quarterly Review of Film and Video. 21 (3): 161–171. doi:10.1080/10509200490437817. S2CID 191583864.
- ^ St John, Graham. "Neotrance and the Psychedelic Festival." Dancecult: Journal of Electronic Dance Music Culture, 1(1) (2009).
- ^ Hirschfelder A (Jan 14, 2016). "The Trips Festival explained". Experiments in Environment: The Halprin Workshops, 1966–1971.
- ^ Rucker JJ (2015). "Psychedelic drugs should be legally reclassified so that researchers can investigate their therapeutic potential". British Medical Journal. 350: h2902. doi:10.1136/bmj.h2902. PMID 26014506. S2CID 46510541.
- ^ "U.S.C. Title 21 – FOOD AND DRUGS". www.govinfo.gov. Retrieved 28 February 2022.
- ^ Harrington A (2023-11-01). "Mental Health's Stalled (Biological) Revolution: Its Origins, Aftermath & Future Opportunities". Daedalus. 152 (4): 166–185. doi:10.1162/daed_a_02037. ISSN 0011-5266.
- ^ "Pot Prohibition Continues Collapsing, and Psychedelic Bans Could Be Next". November 9, 2022.
- ^ "New Hampshire Lawmakers File Psilocybin And Broader Drug Decriminalization Bills For 2022". December 29, 2021.
- ^ Tuccille J (3 August 2022). "Scofflaws Lead the Way To Legalizing Psychedelic Drugs". Reason. Retrieved 18 January 2023.
Further reading
[edit]- Echard W (2017). Psychedelic Popular Music: A History through Musical Topic Theory. Bloomington, IN: Indiana University Press. doi:10.2307/j.ctt1zxxzgx. ISBN 978-0-253-02645-3.
- Halberstadt AL, Franz X. Vollenweider, David E. Nichols, eds. (2018). Behavioral Neurobiology of Psychedelic Drugs. Vol. 36. Berlin, Heidelberg: Springer. ISBN 978-3-662-55878-2.
- Jay M (2019). Mescaline: A Global History of the First Psychedelic. New Haven, CT: Yale University Press. doi:10.2307/j.ctvgc61q9. ISBN 978-0-300-25750-2. S2CID 241952235.
- Letheby C (2021). Philosophy of Psychedelics. Oxford: Oxford University Press. doi:10.1093/med/9780198843122.001.0001. ISBN 978-0-19-884312-2.
- Richards WA (2016). Sacred Knowledge: Psychedelics and Religious Experiences. New York: Columbia University Press. ISBN 978-0-231-54091-9.
- Siff S (2015). Acid Hype: American News Media and the Psychedelic Experience. Champaign, Illinois: University of Illinois Press. ISBN 978-0-252-09723-2.
- Winstock, Ar; Timmerman, C; Davies, E; Maier, Lj; Zhuparris, A; Ferris, Ja; Barratt, Mj; Kuypers, Kpc (2021). Global Drug Survey (GDS) 2020 Psychedelics Key Findings Report.
External links
[edit]Psychedelic Timeline by Tom Frame, Psychedelic Times
